Antibody engineering focuses on intentional alterations to immunoglobulin genetics and architecture to amplify functional performance. These adaptive immune system-derived proteins naturally execute antigen recognition and neutralization through precise molecular targeting. The field now occupies a strategic position in biomedical innovation, facilitating development of advanced therapeutic agents, precision diagnostics, and specialized investigative methodologies. By addressing inherent constraints of native antibodies, this approach allows customization for specialized applications, broadening therapeutic intervention strategies and experimental capabilities in biological sciences.
 Fig.1 Overview of monoclonal antibodies and their variants.1,3
Fig.1 Overview of monoclonal antibodies and their variants.1,3
Goals of Engineering Modification
- Affinity enhancement: Amplifying paratope-epitope interaction kinetics to improve target engagement efficacy.
- Specificity redirection: Reprogramming antigen recognition domains to either novel targets or distinct epitopes.
- Immunogenicity reduction: Humanizing xenogeneic frameworks (e.g., murine-derived antibodies) to mitigate host immune reactions.
- Multifunctionalization: Designing antibodies with dual targeting capabilities or hybrid effector functions (e.g., bispecific engagement platforms).
- Stability improvement: Augmenting resistance to thermal/chemical denaturation for improved storage stability and in vivo persistence.
- Pharmacokinetic optimization: Modulating circulatory half-life and biodistribution patterns through Fc domain engineering.
Effector potentiation: Fine-tuning immune recruitment capacity (e.g., ADCC amplification or CDC modulation) via glycoengineering approaches.
Major Antibody Engineering Technologies and Strategies
Phage Display
This technology employs bacteriophage vectors to express antibody fragments on viral surfaces. Genetic sequences encoding antibodies are incorporated into phage genomes, enabling phenotypic linkage between genotype and binding capacity. Iterative panning against immobilized antigens enriches high-affinity clones through selective pressure. The approach facilitates rapid isolation of human-derived binders, particularly against challenging targets refractory to conventional immunization protocols. Its versatility extends to affinity optimization and novel epitope discovery.
 Fig.2 Phage display process.2,3
Fig.2 Phage display process.2,3
Hybridoma Technology
The classical method involves fusing antigen-primed murine splenocytes with immortalized myeloma partners. Resultant hybrid cells perpetually secrete monoclonal antibodies of defined specificity. Critical stages include animal immunization, cell fusion, hypoxanthine-aminopterin-thymidine (HAT) selection, and clonal expansion. Despite emerging alternatives, this technique remains foundational for research-grade mAb production, maintaining utility in diagnostic assay development.
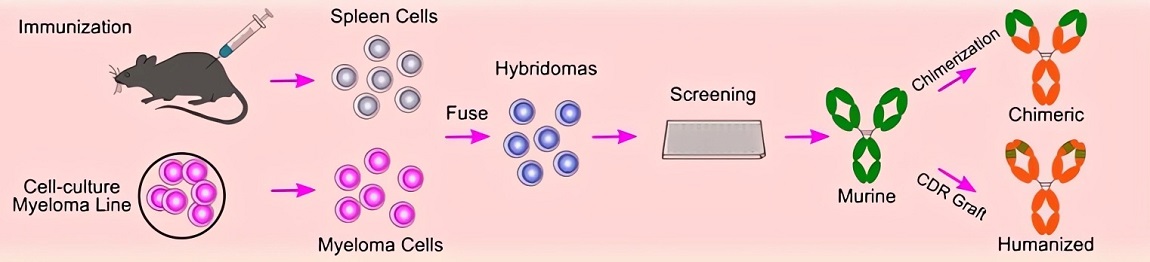 Fig.3 Mouse hybridoma technique.2,3
Fig.3 Mouse hybridoma technique.2,3
Display Platform Variations
Alternative display systems circumvent phage limitations. Yeast surface engineering couples antibody expression with eukaryotic secretion machinery, enabling fluorescence-activated sorting for stability-enhanced variants. Ribosome display operates in cell-free environments, tethering nascent polypeptides to their mRNA templates during in vitro translation. This method permits library diversities exceeding biological constraints. Bacterial and mammalian cell displays offer additional options for context-specific screening.
Affinity Maturation Techniques
Evolution-inspired strategies enhance binding kinetics. Chain shuffling recombines heavy/light chain pairings while maintaining target engagement. Saturation mutagenesis targets complementarity-determining regions (CDRs) to explore sequence space. DNA shuffling and codon-based mutagenesis further diversify variable domains. High-throughput screening identifies variants with improved antigen recognition profiles.
Humanization and Human Antibody Technologies
Xenogeneic antibody mitigation strategies progress through sequential refinement. Chimeric constructs retain murine variable domains fused to human constant regions. CDR grafting transplants murine hypervariable loops onto human framework scaffolds. Transgenic animal platforms and synthetic human libraries yield fully human sequences, circumventing immunogenicity through species-matched architectures.
Fragment Engineering
Proteolytic or recombinant generation of antibody subdomains addresses full-length limitations. Fab fragments preserve antigen recognition while eliminating Fc-mediated effects. Single-chain variable fragments (scFvs) link VH and VL domains via flexible peptides, enhancing tissue permeability. Domain antibodies (dAbs) utilize isolated variable domains for compact targeting modules. These derivatives exhibit improved pharmacokinetics and manufacturing feasibility.
Bispecific and Multispecific Antibodies
Bispecific architectures enable simultaneous antigen recognition through heterologous binding sites. Quadroma technology fuses two hybridomas, while recombinant approaches engineer controlled chain pairing. T-cell redirecting formats bridge malignant cells and immune effectors. Multispecific variants coordinate complex target interactions, enabling pathway modulation and precision payload delivery.
Antibody-Drug Conjugates
Antibody-drug conjugates (ADCs) merge tumor-specific targeting with potent cytotoxins. Site-specific conjugation technologies ensure defined drug-antibody ratios. Cleavable linkers balance circulation stability and intracellular payload release. Advanced iterations employ enzymatically cleavable systems or antibody-directed prodrug activation, expanding therapeutic indices in oncology applications.
Applications of Antibody Engineering
Antibody engineering demonstrates extensive utility across multiple disciplines:
Drug Development
Reconfigured antibodies dominate biologic drug pipelines, particularly in oncology (malignant cell targeting, proliferative pathway inhibition, immune potentiation, cytotoxic payload delivery), autoimmune management (cytokine/receptor neutralization in rheumatoid pathologies), and antimicrobial strategies (pathogen inactivation). Bispecific constructs exhibit clinical potential through dual-target engagement in immunomodulatory regimens.
Diagnostic Enhancement
Precision-engineered variants elevate assay performance via two mechanisms:
- Discriminatory capacity: Exclusive recognition of low-abundance biomarkers through binding optimization
- Adaptability: Fragment-derived formats (scFv, Fab) compatible with multiplex platforms, including fluorescence-activated sorting, automated immunoassays, and tissue microarray analysis
Biotechnology Research
Customized antibodies enable molecular interrogation through three primary modalities:
- Protein manipulation: Selective capture, functional modulation, or post-translational modification analysis.
- Signaling dissection: Ligand-receptor interaction mapping via agonistic/antagonistic constructs.
- Cellular profiling: High-resolution antigen localization through advanced immunohistochemical protocols and multiparametric flow cytometric characterization.
Design and Optimization Considerations in Antibody Engineering
The strategic development of therapeutic antibodies demands systematic evaluation of interdependent variables across multiple domains:
Target Selection and Validation: Effective engineering begins with rigorous identification of disease-associated molecular targets exhibiting both biological significance and therapeutic accessibility. Validation protocols employ orthogonal methodologies, including genetic perturbation studies, preclinical disease modeling, and functional pathway analyses to establish causal relationships between target modulation and clinical outcomes.
Structure-Function Relationship: Empirical optimization relies on deciphering molecular interactions between antibody architecture and biological activity. Critical binding residues are mapped through combinatorial mutagenesis, while biophysical techniques like cryo-electron microscopy and molecular dynamics simulations reveal how conformational alterations influence functional parameters such as target avidity, thermal resilience, and Fcγ receptor engagement.
Pharmacokinetics and Pharmacodynamics: Therapeutic performance is modulated through deliberate engineering of absorption, distribution, and elimination profiles. Fragment antigen-binding (Fab) modifications alter tissue penetration rates, whereas crystallizable fragment (Fc) glycoengineering impacts serum half-life via neonatal Fc receptor recycling. Concurrently, pharmacodynamic tailoring ensures optimal target occupancy dynamics and downstream effector cell recruitment.
Immunogenicity Assessment and Reduction Strategies: Sequence optimization employs hierarchical deimmunization approaches combining structural homology modeling with epitope depletion algorithms. Humanization protocols systematically graft complementarity-determining regions onto human framework scaffolds, while transgenic platforms enable production of fully human antibodies. Immunogenic potential is prospectively evaluated through major histocompatibility complex binding assays and epitope similarity screening against immune repertoire databases.
Production Processes and Quality Control: Manufacturing challenges necessitate integrated process development addressing translational fidelity between discovery-scale and commercial production. Critical quality attributes encompassing aggregation propensity, post-translational modification patterns, and batch-to-batch consistency are monitored through orthogonal analytical methods, including mass spectrometry, capillary electrophoresis, and bioactivity potency assays. Process validation ensures robust control over critical parameters affecting drug substance stability and therapeutic performance.
References
- Sharma, Prerna, et al. "Therapeutic antibodies in medicine." Molecules 28.18 (2023): 6438.
- Wang, Zeng, et al. "Development of therapeutic antibodies for the treatment of diseases." Molecular biomedicine 3.1 (2022): 35.
- Distributed under Open Access license CC BY 4.0, without modification.


 Fig.1 Overview of monoclonal antibodies and their variants.1,3
Fig.1 Overview of monoclonal antibodies and their variants.1,3 Fig.2 Phage display process.2,3
Fig.2 Phage display process.2,3 Fig.3 Mouse hybridoma technique.2,3
Fig.3 Mouse hybridoma technique.2,3

