Antibody conjugation is the chemical process of covalently linking an antibody to another molecule, known as a "payload" or "reporter." This strategic union creates a bifunctional tool, combining the antibody's exquisite specificity with the unique properties of the conjugated molecule. This technique is indispensable in biomedical research and diagnosis, enabling targeted delivery, enhanced detection, and precise quantification of biological targets, ultimately accelerating scientific understanding and therapeutic development.
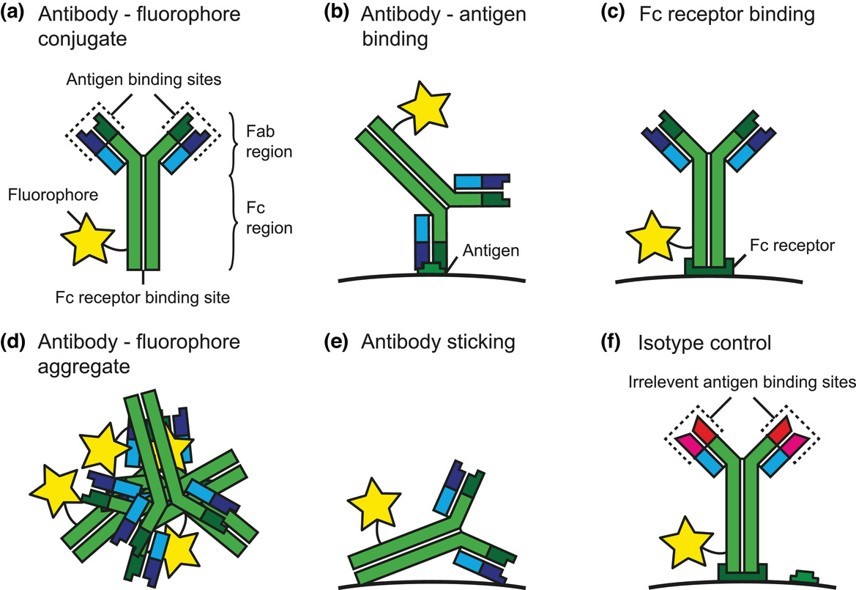 Fig.1 Schematic representation of a fluorescent antibody conjugate and processes involved in antibody staining.1
Fig.1 Schematic representation of a fluorescent antibody conjugate and processes involved in antibody staining.1
Antibody Conjugation Technology
NHS (N-Hydroxysuccinimide) Ester Method
This widely utilized method targets primary amine groups (lysine residues) on the antibody. While versatile and straightforward, it can result in heterogeneous products due to multiple potential conjugation sites, requiring careful optimization to preserve antibody function.
Isothiocyanate Method: Primarily used for conjugating fluorophores, this method also reacts with primary amine groups. It forms stable thiourea linkages, making it a popular choice for fluorescent labeling, though it shares similar heterogeneity considerations with NHS ester chemistry.
Carbodiimide Method
Carbodiimides, such as 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), facilitate the formation of amide bonds between carboxyl groups (on one molecule) and amine groups (on another). This method is highly adaptable for conjugating various molecules, including proteins and peptides, by activating carboxyl groups.
Periodate Method
This technique specifically targets carbohydrate moieties (glycans) present on the antibody's Fc region. Oxidation with periodate generates reactive aldehyde groups, which can then be coupled with hydrazide or amine-containing payloads. This method offers excellent site-specificity, preserving the antibody's antigen-binding site.
Maleimide Chemistry (Cysteine-Based Conjugation)
Maleimide-based reactions specifically target free sulfhydryl (thiol) groups, typically found on cysteine residues. This chemistry offers greater site-specificity compared to amine-reactive methods, especially when antibodies are engineered to contain specific cysteine residues, leading to more homogeneous conjugates.
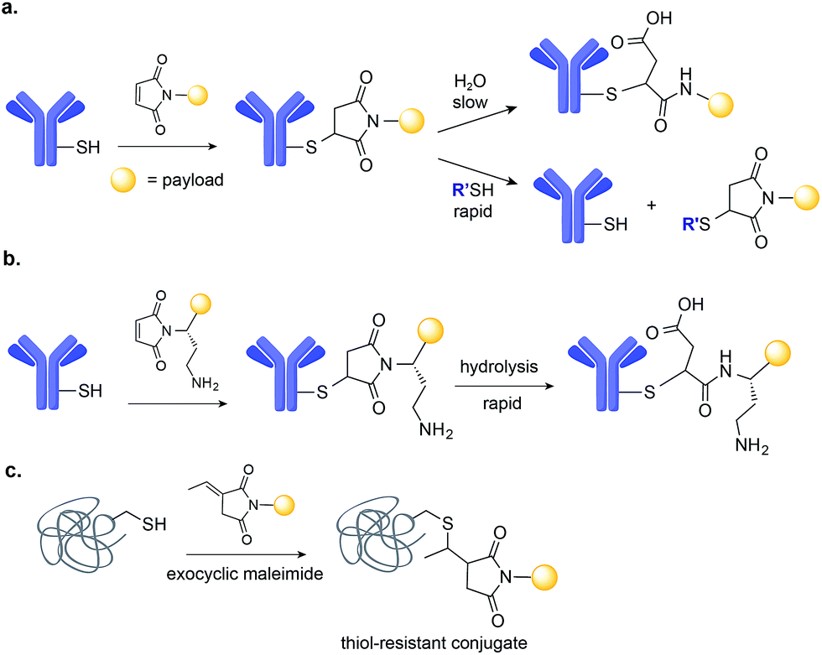 Fig.2 Maleimide-based conjugation.2
Fig.2 Maleimide-based conjugation.2
Site-Specific Conjugation
These methods ensure that the payload attaches to a predetermined location on the antibody, often away from the antigen-binding site. This approach maximizes the preservation of antibody affinity and minimizes functional impairment, leading to superior, more consistent conjugates.
Commonly Used Labels
The choice of marker or label is critical, dictating the conjugate's function and application. We offer conjugation with a wide spectrum of functional tags:
Fluorophores: Fluorescent dyes are indispensable for visualizing biological processes. Common examples include fluorescein isothiocyanate (FITC), phycoerythrin (PE), and cyanine (Cy) dyes (e.g., Cy2, Cy3, Cy5). Each possesses distinct spectral characteristics (excitation and emission wavelengths), enabling multiplexed detection. They are widely applied in flow cytometry for cell phenotyping and sorting, immunofluorescence for cellular and tissue imaging, and Western Blot for protein detection.
Enzymes: Enzymes serve as highly sensitive reporters, converting a substrate into a detectable signal. Horseradish peroxidase (HRP) and alkaline phosphatase (AP) are the most common. HRP, with its robust activity and multiple lysine residues that can be modified without affecting enzyme function, is ideal for conjugation. These enzyme conjugates are extensively used in enzyme-linked immunosorbent assays (ELISA) and immunohistochemistry, generating colorimetric, chemiluminescent, or fluorescent signals for detection.
Biotin: Biotinylation of antibodies is a powerful tool for signal amplification, leveraging the extraordinarily high affinity between biotin and streptavidin (or avidin). A biotinylated antibody can be detected by multiple streptavidin-conjugated reporter molecules (e.g., streptavidin-HRP or streptavidin-fluorophore), significantly enhancing the signal intensity and sensitivity of an assay.
Magnetic Beads: Antibodies conjugated to magnetic beads provide a convenient and efficient method for isolating specific targets. These conjugates are invaluable in applications such as immunoprecipitation for protein isolation, cell sorting for enriching specific cell populations, and nucleic acid purification, allowing for rapid and gentle separation using a magnetic field.
Nanoparticles: Nanoparticles, including colloidal gold and quantum dots, offer unique optical and electronic properties for highly sensitive detection. Colloidal gold conjugates are foundational in rapid diagnostic tests (e.g., lateral flow assays) due to their visual detection capabilities. Quantum dots provide bright, photostable fluorescence with narrow emission spectra, enabling multiplexed, high-sensitivity detection in various immunoassay formats.
Other Tags: Beyond the widely used markers, antibodies can also be conjugated to other specialized tags. These include radioactive isotopes (e.g., Iodine-125) for high-sensitivity detection in radioimmunoassays or in vivo imaging, and bioluminescent proteins (e.g., Luciferase) for ELISAs and in vivo imaging applications where light emission is desired.
Application of Antibody Conjugation
Targeted Drug Delivery
Antibody-drug conjugates (ADCs) exemplify this application. By linking highly potent cytotoxic drugs to antibodies that specifically recognize cancer cells, ADCs deliver therapeutic payloads directly to tumors, minimizing systemic toxicity and improving treatment efficacy for various cancers. This precision delivery is also being explored for autoimmune and infectious diseases.
Imaging
Conjugated antibodies are indispensable tools for visualizing biological structures and processes. Whether labeled with fluorescent dyes for microscopy and flow cytometry, or with radioisotopes for in vivo molecular imaging, they enable researchers to precisely locate and track specific molecules, cells, or pathogens within complex biological systems.
Immunoassay
The vast majority of modern immunoassay techniques rely on conjugated antibodies for sensitive and specific detection. These assays are fundamental for diagnosing diseases, monitoring treatment efficacy, detecting pathogens, and quantifying biomarkers in research and clinical settings, providing accurate and reliable results.
| Immunoassay Technique |
Conjugate Label |
Application |
| ELISA |
HRP/AP, biotin |
Direct, indirect, and sandwich ELISA for antigen/antibody detection and signal amplification |
| Western Blot |
HRP/AP, fluorescent dyes, biotin |
Specific detection of proteins separated by gel electrophoresis |
| Immunofluorescence |
Fluorophores |
Accurate localization and visualization of target molecules within cells or tissue sections. |
| Flow Cytometry |
Fluorophores |
Quantitative analysis of cell surface or intracellular proteins and precise cell sorting |
| Immunohistochemistry |
HRP/AP, biotin |
Detection of target antigens directly within tissue sections for diagnosis and biomarker identification |
| Immunoprecipitation |
Magnetic beads |
Selective isolation of specific proteins or complexes from mixtures for downstream analysis |
Quality Control and Optimization of Conjugated Antibodies
Quality Assessment
Key indicators for assessing conjugated antibody quality include:
- Coupling efficiency: This measures the percentage of antibody successfully conjugated to the marker, indicating the yield of the conjugation reaction.
- Antibody activity: Crucially, the antibody must retain its antigen-binding ability after conjugation. This is typically assessed through functional assays like ELISA or flow cytometry.
- Marker activity: The conjugated marker (e.g., enzyme, fluorescent dye) must also maintain its full functionality. For enzymes, this means preserving catalytic activity; for fluorophores, it means stable and bright fluorescence.
- Batch-to-batch consistency: For both research and clinical applications, ensuring stable product quality and performance across different manufacturing batches is critical for reproducibility and reliability.
Optimization Strategy
Optimizing the conjugation process is vital to achieve high-quality conjugates. Considerations include:
- Antibody concentration: The starting concentration of the antibody can significantly impact conjugation efficiency.
- Molar ratio of marker to antibody: This ratio directly influences the Drug-to-Antibody Ratio (DAR) and homogeneity of the conjugate.
- Reaction time: The duration of the conjugation reaction affects yield and potential over-conjugation.
- pH value: Optimal pH is crucial for the efficiency and specificity of different conjugation chemistries.
- Purification method: Effective purification (e.g., size exclusion chromatography, dialysis) is essential to remove unconjugated markers and aggregates, ensuring a pure and functional product.
Why Choose Us
- Professional Technology and Experience
With years of professional experience in biomedicine, our deep scientific understanding and technical prowess ensure the delivery of high-caliber antibody conjugates, precisely tailored to your needs.
- Product Advantages
- High conjugation efficiency and uniformity
- High activity after conjugation
- Low background noise
- Strict quality control
- Wide selection of markers
- Customized conjugation services
- Customer Support
Our dedicated customer support team offers expert technical assistance and consulting services, guiding you from initial concept to successful application.
References
- Welsh, Joshua A., et al. "A compendium of single extracellular vesicle flow cytometry." Journal of extracellular vesicles 12.2 (2023): e12299. Distributed under Open Access license CC BY 4.0, without modification.
- Akkapeddi, Padma, et al. "Construction of homogeneous antibody–drug conjugates using site-selective protein chemistry." Chemical Science 7.5 (2016): 2954-2963. Distributed under Open Access license CC BY 3.0, without modification.


 Fig.1 Schematic representation of a fluorescent antibody conjugate and processes involved in antibody staining.1
Fig.1 Schematic representation of a fluorescent antibody conjugate and processes involved in antibody staining.1 Fig.2 Maleimide-based conjugation.2
Fig.2 Maleimide-based conjugation.2

